scAutoQC workflow tutorial

[1]:
import scanpy as sc
import sctk
sc.settings.set_figure_params(dpi=80)
This notebook will show how to apply SCTK’s automatic QC workflow, scAutoQC. The process has four steps:
sctk.calculate_qc()computes QC measuressctk.cellwise_qc()performs cell-level QC callingsctk.generate_qc_clusters()creates QC-space clusters of cellssctk.clusterwise_qc()computes cluster-wide statistics of cell quality
It is recommended to run scAutoQC on a per-sample basis. The workflow offers a utility function sctk.multi_resolution_cluster_qc() to attempt clustering with multiple resolutions and draw broader automated conclusions, which is likely to be of use when faced with multiple samples to process. This leads to a simplified analysis flow of:
sctk.calculate_qc()
sctk.cellwise_qc()
sctk.multi_resolution_cluster_qc()
Let’s take a closer look at what each step in the process does, using Scanpy’s PBMC object as input.
[2]:
adata = sc.datasets.pbmc3k()
adata
[2]:
AnnData object with n_obs × n_vars = 2700 × 32738
var: 'gene_ids'
This object features raw counts for some PBMC data, and nothing else for now. We can use sctk.calculate_qc() to automatically flag some common technical features (the defaults are human mitochondrial, ribosomal and hemoglobin genes) and subsequently use sc.pp.calculate_qc_metrics() to compute their percentages in each cell, along with standard measures like the number of genes and counts.
[3]:
sctk.calculate_qc(adata)
adata
[3]:
AnnData object with n_obs × n_vars = 2700 × 32738
obs: 'n_counts', 'log1p_n_counts', 'n_genes', 'log1p_n_genes', 'percent_mito', 'n_counts_mito', 'percent_ribo', 'n_counts_ribo', 'percent_hb', 'n_counts_hb', 'percent_top50'
var: 'gene_ids', 'mito', 'ribo', 'hb', 'n_counts', 'n_cells'
You can see the .obs of the object populated with the QC measures we just generated.
The second step of the scAutoQC workflow involves determining which of the cells pass QC individually.
[4]:
sctk.cellwise_qc(adata)
adata
n_counts: [959.4421576429211, 15844.0029296875], 2563/2700 passed
n_genes: [320.5358429786537, 3421.998779296875], 2678/2700 passed
percent_mito: [0.0, 5.943145075272779], 2674/2700 passed
percent_ribo: [13.568207997582016, 51.07162303563632], 2627/2700 passed
WARNING: Failed to find lower bound, using min value instead.
percent_hb: [0.0, 0.002321736026317521], 2375/2700 passed
2184/2700 pass
[4]:
AnnData object with n_obs × n_vars = 2700 × 32738
obs: 'n_counts', 'log1p_n_counts', 'n_genes', 'log1p_n_genes', 'percent_mito', 'n_counts_mito', 'percent_ribo', 'n_counts_ribo', 'percent_hb', 'n_counts_hb', 'percent_top50', 'cell_passed_qc'
var: 'gene_ids', 'mito', 'ribo', 'hb', 'n_counts', 'n_cells'
uns: 'scautoqc_ranges'
There’s now a cell_passed_qc column in .obs which features per-cell QC calls.
[5]:
adata.obs['cell_passed_qc'].sum()
[5]:
2184
There’s also adata.uns['scautoqc_ranges'] which captures the inferred range print-out displayed as the function runs.
[6]:
adata.uns['scautoqc_ranges']
[6]:
| low | high | |
|---|---|---|
| n_counts | 959.442158 | 15844.00293 |
| n_genes | 320.535843 | 3421.998779 |
| percent_mito | 0.0 | 5.943145 |
| percent_ribo | 13.568208 | 51.071623 |
| percent_hb | 0.0 | 0.002322 |
Controlling the desired QC thresholds requires a bit of explanation.
Under the hood, sctk.cellwise_qc() models each of the specified QC metrics as a Gaussian mixture model, with the model proposing possible low and high value cutoffs at points where the probability density function of the mixture falls below a threshold (0.05 by default). The function is written to allow robust filtering, requiring a specific formatting of metrics. The default values are stored in sctk.default_metric_params_df:
[7]:
sctk.default_metric_params_df
[7]:
| min | max | scale | side | min_pass_rate | |
|---|---|---|---|---|---|
| n_counts | 1000.00 | NaN | log | min_only | 0.10 |
| n_genes | 100.00 | NaN | log | min_only | 0.10 |
| percent_mito | 0.01 | 20.0 | log | max_only | 0.10 |
| percent_ribo | 0.00 | 100.0 | log | both | 0.10 |
| percent_hb | NaN | 1.0 | log | max_only | 0.10 |
| percent_soup | NaN | 5.0 | log | max_only | 0.10 |
| percent_spliced | 50.00 | 97.5 | log | both | 0.10 |
| scrublet_score | NaN | 0.3 | linear | max_only | 0.95 |
The variable is a data frame, with rows named after computed QC metrics present in the .obs of the input object. Values absent from .obs will get skipped (so in our case, percent_soup, percent_spliced and scrublet_score all got bypassed when we ran the function). The five columns control the input to the GMM and subsequent filtering behaviour:
``min``: the minimum value to use in modelling the distribution; values lower than this will be skipped; set to
np.nanto use the encountered minimum``max``: the maximum value to use in modelling the distribution; values higher than this will be skipped; set to
np.nanto use the encountered maximum``scale``:
"log"to log-transform the QC metric values prior to modelling,"linear"to keep the values un-transformed``side``:
"min_only"to only perform filtering of low values,"max_only"to only perform filtering of high values,"both"to perform filtering from both sides``min_pass_rate``: the minimum proportion of cells that need to pass the filtering on this feature; if the fraction is too low, the GMM is discarded and the QC values are thresholded based on the provided minimum/maximum
As the function goes along, it prints out the actual QC metric value ranges it used in filtering, and how many cells fall within that range. For example, notice how we specified a 100-gene minimum for the modelling, but the proposed data-driven cutoff ends up being 320.
Let’s try out modifying the metrics values by taking the default data farme, removing the three measures absent from our object, and increasing the n_genes minimum to 500.
[8]:
metrics = sctk.default_metric_params_df.loc[["n_counts",
"n_genes",
"percent_mito",
"percent_ribo",
"percent_hb"], :]
metrics.loc["n_genes", "min"] = 500
metrics
[8]:
| min | max | scale | side | min_pass_rate | |
|---|---|---|---|---|---|
| n_counts | 1000.00 | NaN | log | min_only | 0.1 |
| n_genes | 500.00 | NaN | log | min_only | 0.1 |
| percent_mito | 0.01 | 20.0 | log | max_only | 0.1 |
| percent_ribo | 0.00 | 100.0 | log | both | 0.1 |
| percent_hb | NaN | 1.0 | log | max_only | 0.1 |
Let’s re-run sctk.cellwise_qc() with these parameters.
[9]:
sctk.cellwise_qc(adata, metrics=metrics)
n_counts: [959.4421576429211, 15844.0029296875], 2563/2700 passed
n_genes: [495.3027116195289, 3421.998779296875], 2486/2700 passed
percent_mito: [0.0, 5.943145075272779], 2674/2700 passed
percent_ribo: [13.568207997582016, 51.07162303563632], 2627/2700 passed
WARNING: Failed to find lower bound, using min value instead.
percent_hb: [0.0, 0.002321736026317521], 2375/2700 passed
2118/2700 pass
The gene count filtering is different now, as expected.
The third step of the QC workflow is to cluster the cells based on specified metrics, performing a quick Scanpy analysis using the metrics as features. This creates groups of cells that have similar QC profiles.
[10]:
#present as columns in obs of the object
metrics_list = ["log1p_n_counts", "log1p_n_genes", "percent_mito", "percent_ribo", "percent_hb"]
sctk.generate_qc_clusters(adata, metrics = metrics_list)
adata
/home/jovyan/my-conda-envs/sctk/lib/python3.9/site-packages/tqdm/auto.py:21: TqdmWarning: IProgress not found. Please update jupyter and ipywidgets. See https://ipywidgets.readthedocs.io/en/stable/user_install.html
from .autonotebook import tqdm as notebook_tqdm
[10]:
AnnData object with n_obs × n_vars = 2700 × 32738
obs: 'n_counts', 'log1p_n_counts', 'n_genes', 'log1p_n_genes', 'percent_mito', 'n_counts_mito', 'percent_ribo', 'n_counts_ribo', 'percent_hb', 'n_counts_hb', 'percent_top50', 'cell_passed_qc', 'qc_cluster'
var: 'gene_ids', 'mito', 'ribo', 'hb', 'n_counts', 'n_cells'
uns: 'scautoqc_ranges'
obsm: 'X_umap_qc'
There’s now an .obs column called qc_cluster, and an embedding called X_umap_qc. Let’s take a look!
[11]:
sc.pl.embedding(adata, "X_umap_qc", color=["qc_cluster", "log1p_n_counts"], color_map="OrRd")
/home/jovyan/my-conda-envs/sctk/lib/python3.9/site-packages/scanpy/plotting/_tools/scatterplots.py:394: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
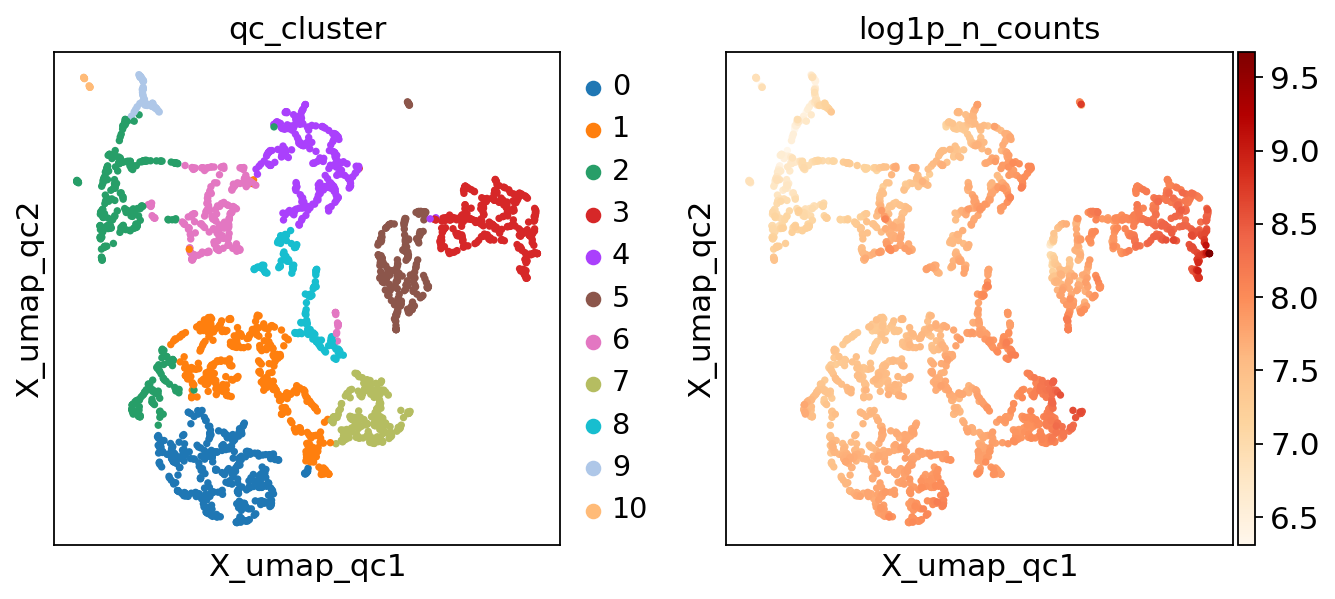
The final step of the scAutoQC workflow determines computes a fraction of passing cells for each cluster. If a cluster has a high enough fraction of passing cells (controlled via the threshold parameter, default 0.5), it’s deemed to be a good QC cluster.
You can use your own per-cell QC calls here rather than the ones provided by sctk.cellwise_qc() if desired, just specify which .obs column to take via the cell_qc_key argument.
[12]:
sctk.clusterwise_qc(adata)
adata
[12]:
AnnData object with n_obs × n_vars = 2700 × 32738
obs: 'n_counts', 'log1p_n_counts', 'n_genes', 'log1p_n_genes', 'percent_mito', 'n_counts_mito', 'percent_ribo', 'n_counts_ribo', 'percent_hb', 'n_counts_hb', 'percent_top50', 'cell_passed_qc', 'qc_cluster', 'cluster_passed_qc'
var: 'gene_ids', 'mito', 'ribo', 'hb', 'n_counts', 'n_cells'
uns: 'scautoqc_ranges', 'qc_cluster_colors'
obsm: 'X_umap_qc'
There’s now a cluster_passed_qc column. Let’s take a look how it compares to the cell level QC.
[13]:
#this won't be necessary in scanpy 1.10.0, booleans will become directly plottable
for col in ['cell_passed_qc', 'cluster_passed_qc']:
adata.obs[col+"_int"] = adata.obs[col].astype(int)
sc.pl.embedding(adata, "X_umap_qc", color=["cell_passed_qc_int", "cluster_passed_qc_int"])
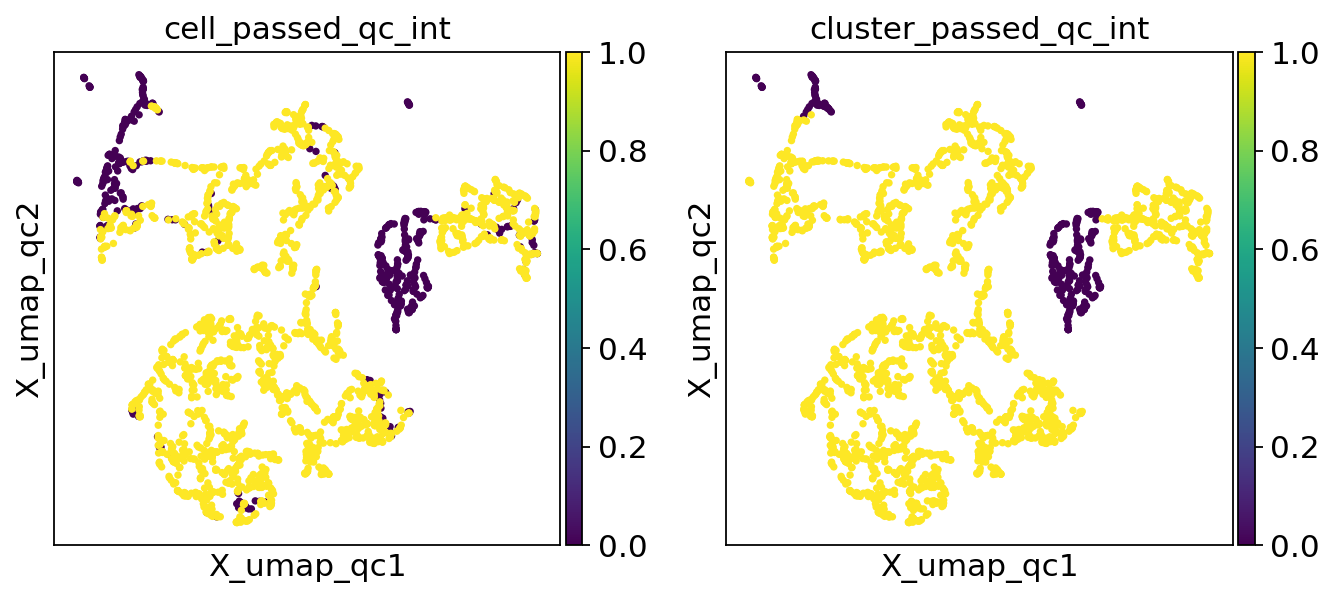
We seem to get a nontrivial number of cells individually flagged as poor quality colocating in the top left of the plot, but only a small subset of them remain after cluster membership is leveraged. This can be overcome by changing the clustering resolution, but it’s a process that requires a degree of oversight. While not problematic when operating on a single sample in a demo notebook, this would be quite laborious and tedious when processing multiple samples ahead of an analysis.
To combat this issue, scAutoQc comes with sctk.multi_resolution_cluster_qc(). The function tries out multiple different clustering resolutions (by default 0.1 to 1, going up in 0.1 intervals) and proposes two sets of QC calls, leveraging the information it collects along the way: - a single selected resolution where the Jaccard index between cell-level and cluster-level QC calls is the highest; by default cells/clusters failing QC are compared as they are likely to be the smaller set - the
fraction of clusterings where the cell passes QC, subsequently thresholded
In practice, you can run this function directly after sctk.cellwise_qc(), skipping sctk.generate_qc_clusters() and sctk.clusterwise_qc(). The two sets of QC calls are added in cluster_passed_qc and consensus_passed_qc respectively.
[14]:
sctk.multi_resolution_cluster_qc(adata, metrics = metrics_list)
#this won't be necessary in scanpy 1.10.0, booleans will become directly plottable
for col in ['cell_passed_qc', 'cluster_passed_qc', 'consensus_passed_qc']:
adata.obs[col+"_int"] = adata.obs[col].astype(int)
sc.pl.embedding(adata, "X_umap_qc", color=["cell_passed_qc_int",
"cluster_passed_qc_int",
"consensus_fraction",
"consensus_passed_qc_int"])
Best overlap found for resolution 0.8
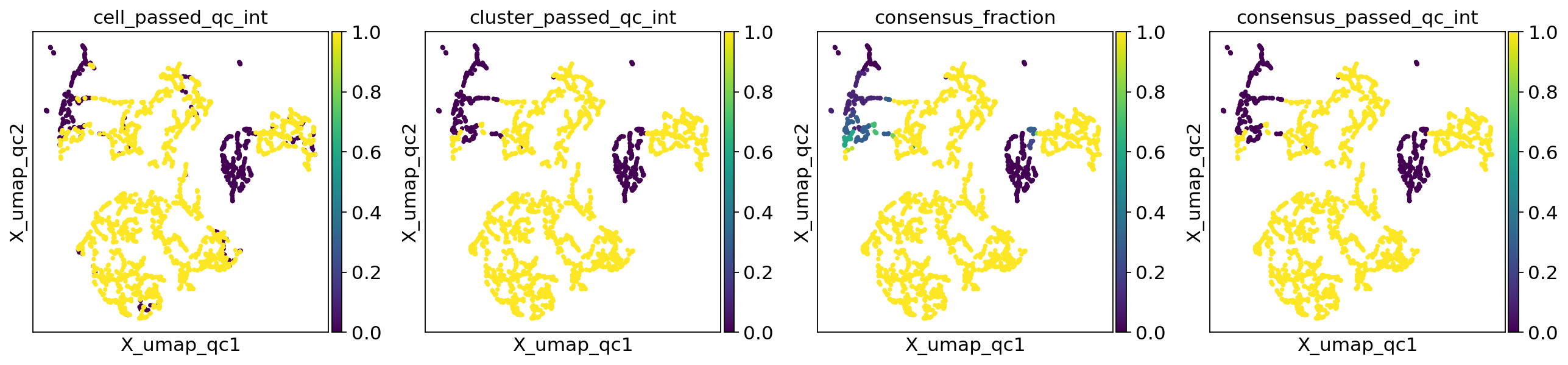
The plots align better now. The two sets of multi-clustering QC calls seem to agree pretty well as well.
At this point you should be able to use the QC workflow on your own data.